Influx

∙ 100% Human Bone:
No carriers, fillers or binding agents.
∙ Osteoinductive Potential:
Processing improves the availability of inductive properties by maximizing the exposure of growth factors and proteins in a
versatile scaffold.
∙ Optimal Handling:
All forms can be hydrated with cBMA or PRP for precise graft placement that is resistant to irrigation.
∙ Osteoconductive:
Novel, small diameter fibers provide an optimal environment for
cell attachment and proliferation.
∙ Advanced Processing:
Enhanced regenerative capacity over particulate DBM.
Influx Fibrant

∙ Longer & Stronger Fibers:
Proprietary Fibrant™ technology creates longer and stronger
fibers™ that preserve the native nano-topography of the bone's
collagen to enhance cell attachment and offer excellent performance.
∙ Novel Forms:
Fibrant is offered in five unique forms to provide surgeons with
the flexible options they need on a case-to-case basis.
∙ Processing with Purpose:
By demineralizing the cortical struts prior to cutting, bone is cut
around the longitudinal plane, resulting in long and strong fiber.
∙ Formlok™ Technology:
Used in three Fibrant forms, Formlok technology adheres fibers
together in the molding phase to increase stability, resist graft
migration, and assure products retain integrity upon hydration

Influx™ SPARC

∙ Ready-to-use:
Indications include use as a ready-to-use bone graft in spinal
procedures.
∙ Equivalency:
Proven to be equivalent to iliac crest autograft in a preclinical
spine fusion model.
∙ Osteoinductive:
Validated processing assures an osteoinductive response.
∙ Exceptional handling:
Cohesive handling that resists irrigation due to robust biocompatible carrier.
Influx™ PLUS

∙ Ready-to-use:
Indications include use as a ready-to-use bone graft in spinal
procedures.
∙ Equivalency:
Proven to be equivalent to iliac crest autograft in a preclinical
spine fusion model.
∙ Osteoinductive:
Validated processing assures an osteoinductive response.
∙ Exceptional handling:
Cohesive handling that resists irrigation due to robust biocompatible carrier.
Influx™ ProteiOS

∙ A Cascade of Growth Factors:
Contains the highest level of naturally derived proteins.
High concentrations of GF include, but are not limited to, BMP-2,
PDGF, TGF-1, and VEGF.
∙ Confidence & Trust:
Terminally sterilized through gamma irradiation and sourced with meticulous donor screening.
No cellular or self-identifying antigens to illicit an immune
response.
∙ Tailored Patient Care:
Unlock new levels of potential within your preferred graft.
Customize the amount added to match your specific needs.
∙ Proven Results:
In MI-TLIF procedures, ProteiOS usage delivered superior fusion
rates as compared to reported fusion rates for autograft and other
advanced orthobiologics products.

EXABONE HA/TCP

Synthetic biphasic biomaterial.
∙ Optimized characteristics:
Matching the internal structure of healthy human cancellous bone.
∙ Can be mixed with autologous or synthetic bone or bone
marrow aspirate: Rapid and homogeneous fluids absorption.
Creates a highly osteoconductive environment boosting bone
regeneration with a controlled resorption.
∙ Available in four convenient forms:Granules, Discs, Blocks and
and Wedges.

EXABONE PASTE

EXABONE Paste is designed for use in a broad range of non-load bearing osseous defects such as: spinal surgery (cage filling), osteotomies, bone cavity and defect filling, metaphyseal fractures and acetabulum reconstruction. EXABONE Paste can be mixed with autologous or synthetic bone or bone marrow aspirate. Optimized sticky viscosity allows a complete and easy filling of the defects, assuring a superior contact at the implant interface and will resist irrigation. The EXABONE Paste nano surface area is 50 to 100 times greater than traditional synthetic bone graft. Moreover, Exabone Paste is a biomimetic, osteostimulative, and easy to use pre-filled putty applicator. In addition, the osteostimulative effects of the hydroxyapatite nano particles encourages osteogenesis. The ultra-high molecular surface area attracts and adsorbs the natural bone growth promoters available at the implant site.
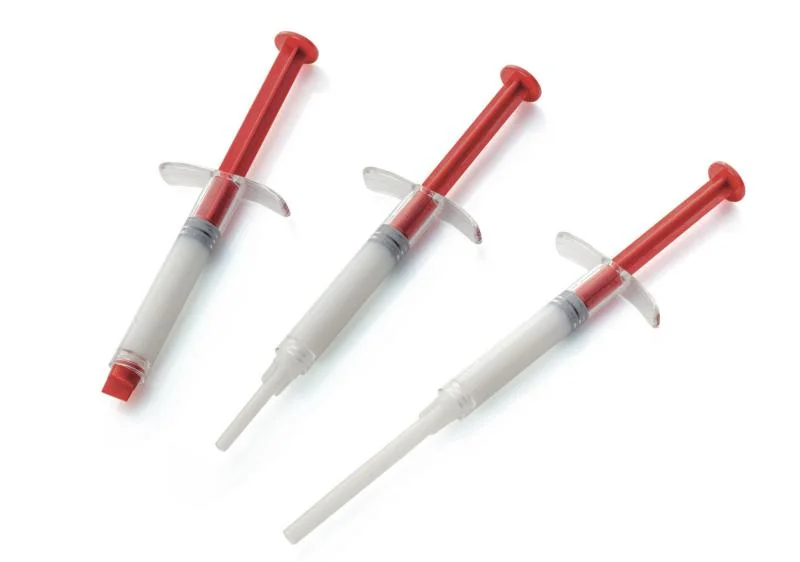
EXABONE PUTTY

EXABONE PUTTY is designed for use in a broad range of non-load bearing osseous defects such as: spinal surgery (cage filling), osteotomies, bone cavity and defect filling, metaphyseal fractures, acetabulum reconstruction. EXABONE PUTTY can be mixed with autologous or synthetic bone or bone marrow aspirate. Advanced technology leads to unique 3 phase specification; Nano HA attracts the biomolecules essential for the recruitment of cells, Micro homogeneous porosity and surface roughness aids cell attachment and nutrient transfer and Macro structured biphasic component supports ingrowth of bone and the regeneration process. EXABONE PUTTY is biomimetic, osteostimulative and ready to use Pre-filled putty applicator.

SYNICEM 1G

Synicem 1G, for manual application is available in two sizes.
• A pouch containing an envelope with 20 g of radiopaque powder
with gentamicine sterilized by ethylene oxide, and an ampoule
with 10 ml of liquid sterilized by ultrafiltration.
• A pouch containing an envelope with 40 g of radiopaque powder
with gentamicine sterilized by ethylene oxide, and an ampoule
with 20 ml of liquid sterilized by ultrafiltration.

SYNICEM 3G

Synicem 3G for syringe application, is available in two sizes.
• A pouch containing an envelope with 40 g of radiopaque powder
with gentamicin sterilized by ethylene oxide, and an ampoule with 20 ml of liquid sterilized by ultrafiltration.
• A pouch containing an envelope with 60 g of radiopaque powder
with gentamicin sterilized by ethylene oxide, and an ampoule with 30 ml of liquid sterilized by ultrafiltration.

SYNIMIX V

REUSABLE INJECTING DEVICE
Reusable injecting device for the Synimix V Mixing and Application
Set.
This reusable Synimed V4 is supplied in a SS sterilization case.
Can be sterilized by any approved hospitalary method. REUSABLE
INJECTING DEVICE.
Reusable injecting device for the Synimix V Mixing and Application
Set.
This reusable Synimed V4 is supplied in a SS sterilization case.
Can be sterilized by any approved hospitalary method.
SYNICEM CRANIOPLASTIE G

RADIOLUCID ACRYLIC CEMENT FOR CRANIOPLASTY WITH GENTAMICIN
Two doses of surgical cement, are supplied in a package which
contains the instructions for use and a PET-G molded blíster,
sealed with Tyvek® containing: 1 flat plastic film tube (Sterile
plastic sleeve), 1 pouch containing 30 gram of powder (polymer)
and 1 glass ampoule with 17 ml of liquid (monomer) protected by a
PET-G thermoformed cradle. The blíster contents are ETO sterilized, while the liquid within the ampoule is sterilized by ultrafiltration.
SYNICEM ISM

CRANIOPLASTY CUSTOM MADE IMPLANTS
Two sterile prostheses and one patient cranial prototype are
supplied, sterilized by EO:
Prosthesis A is supplied in dimensions that match perfectly the bone defect observed in the Computerized Tomography (CT
Helical Scan).
Prosthesis B is 5% larger than the bone defect, for the surgeon to
adapt it according to the intra-operatory findings or to his own
personal preferences.

SYNICEM EVOLUTION

KNEE SPACER WITH ANTIBIOTIC (GENTAMICIN)
∙ A temporary implanted spacer that aids in the treatment of
infected knee prosthesis.
∙ A sterile device, ready to use.
∙ Available in many sizes.
∙ Designed to reach the maximum balance between device and patient, and the fastest application procedure.

SYNICEM SHOULDER SPACER

SHOULDER SPACER WITH ANTIBIOTIC (GENTAMICIN)
Synicem Shoulder Spacer is a temporary implant meant to facilitate the treatment of infected shoulder prosthesis.
Synicem Shoulder spacer with antibiotic is available in a packaging containing the Instructions for use and a double blister containing one spacer sterilized by ETO.
Synicem Shoulder Spacer has completely been manufactured
using bone cement with a high concentration of antibiotic (gentamicin). The Spacer has been reinforced by means of a internal rod made of surgical grade 316L Stainless Steel.

SYNICEM HIP SPACER

HIP SPACER WITH ANTIBIOTIC (GENTAMICIN)
The Synicem Hip Spacer is a premoulded temporary spacer that
aids in the treatment of infected hip prosthesis.
This Synicem Hip Spacer is available in a box with the Instructions for Use leaflet and a ETO sterilized double blister containing one hip spacer.
Synicem Spacer is made of PMMA Bone Cement loaded with high
concentrations of antibiotic (gentamicin). An internal core made of AISI 316L reinforces the device.
KNEE TRIAL PROSTHESES

METALIC TRIAL KNEE SPACERS SET
These devices accessories are trial prostheses for momentary use.
They permit to select the correct size of a definitive spacer without
having to manipulate it. They are made of stainless steel and their
size and shape reproduce those of the corresponding definitive
spacers. They are supplied Non-sterile, inside a multiperforated
box suitable for their sterilization.

SHOULDER TRIAL PROSTHESES

METAL SHOULDER TRIAL PROSTHESIS KIT.
These accessories are trial prostheses for temporary use. They
allow you to choose the right spacer without needing to manipulate it. They are made of stainless steel; they reproduce the shape
and size of the spacers to which they correspond. They are
supplied non-sterile, in a multi-perforated case for sterilization.

HIP TRIAL PROSTHESES

METALLIC HIP TRIAL PROSTHESIS KIT.
These accessories are trial prostheses for temporary use. They
allow you to choose the right spacer without needing to manipulate it. They are made of stainless steel; they reproduce the shape
and size of the spacers to which they correspond. They are
supplied non-sterile, in a multi-perforated case for sterilization.

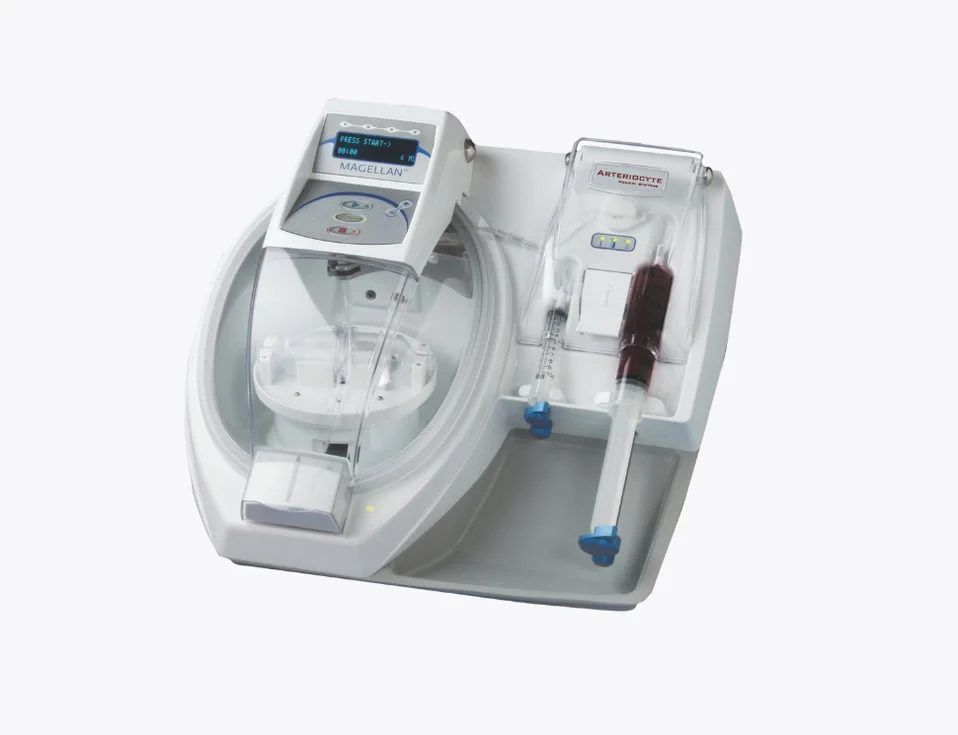
Autologous Therapy Magellan PRP

Fully Automated Decreases user error and provides ease of use. Closed-Loop Design Safer for the patient and the user due to decreased processing steps and minimal breaks in the sterile barrier. Optical Sensor Driven The microprocessor and optical sensors enable Magellan to reliably detect the cellular fraction to gently produce consistent and reproducible cellular concentrations and compositions. Pump-Driven Design Gentle on cells. Selectable Output Allows users to define desired therapeutic volumes (3-10 mL per cycle). Reusable Disposable The Magellan system can process up to three cycles (single patient) using a single disposable, giving options for obtaining both cBMA and PRP while allowing for additional volume and concentration flexibility.